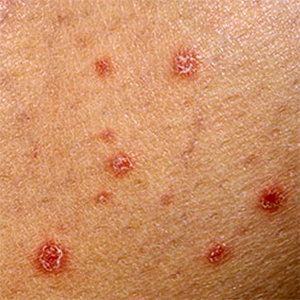
Seven cancer patients receiving guselkumab for treatment of moderate-to-severe psoriasis
HTML: 34
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
To the Editor:
Limited evidence on the use of biologic treatments in psoriatic patients with a history of malignancy is currently available in the literature. The recently published European guidelines recommend the use of anti-TNF, anti-IL17, and anti-23 biological drugs in this special population after discussion case-by-case with a cancer-specialist [...].
Nast A, Smith C, Spuls PI et al. EuroGuiDerm Guideline on the systemic treatment of Psoriasis vulgaris - Part 2: specific clinical and comorbid situations. J Eur Acad Dermatol Venereol 2021;35:281-317. DOI: https://doi.org/10.1111/jdv.16926
Kahn JS, Casseres RG, Her MJ. Treatment of psoriasis with biologics and Apremilast in patients with a history of malignancy: a retrospective chart review. J Drugs Dermatol 2019;18.
Bellinato F, Gisondi P, Maurelli M, Girolomoni G. IL-17A inhibitors in patients with chronic plaque psoriasis and history of malignancy: A case series with systematic literature review. Dermatol Ther 2021;34:e14889. DOI: https://doi.org/10.1111/dth.14889
Chyuan IT, Lai JH. New insights into the IL-12 and IL-23: From a molecular basis to clinical application in immune-mediated inflammation and cancers. Biochem Pharmacol 2020;175:113928. DOI: https://doi.org/10.1016/j.bcp.2020.113928
Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: Results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol 2017;76:418-31. DOI: https://doi.org/10.1016/j.jaad.2016.11.042
Bilal J, Berlinberg A, Bhattacharjee, et al. A systematic review and meta- analysis of the efficacy and safety of the interleukin (IL)-12/23 and IL-17 inhibitors ustekinumab, secukinumab, ixekizumab, brodalumab, guselkumab and tildrakizumab for the treatment of moderate to severe plaque psoriasis. J Dermatolog Treat 2018;29:569-78. DOI: https://doi.org/10.1080/09546634.2017.1422591
Kamiya K, Yamauchi H, Ohtsuki M. Treatment of psoriasis vulgaris with guselkumab in a patient with non-small cell lung cancer. Eur J Dermatol 2020;30:609-11. DOI: https://doi.org/10.1684/ejd.2020.3860
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.





 https://doi.org/10.4081/dr.2022.9282
https://doi.org/10.4081/dr.2022.9282



