Severe and delayed-onset acneiform eruptions as an adverse reaction to regorafenib
HTML: 3
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
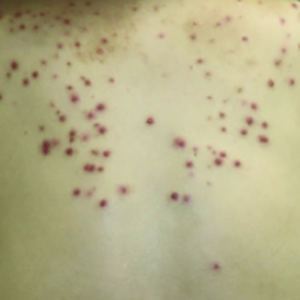
Regorafenib is an oral multikinase inhibitor targeting several tyrosine kinase receptors including BRAF and epidermal growth factor receptor (EGFR) and is approved as a third-line treatment for metastatic gastrointestinal stromal tumor (GIST). While acneiform eruptions have been observed in patients receiving other BRAF and EGFR inhibitors, the commonly reported adverse reactions to regorafenib are fatigue and palmar-plantar erythrodysesthesia. Herein, we report, to the best of our knowledge, the first case who presented with a severe acneiform eruption 24 months after beginning regorafenib for the treatment of GIST. A 61-year-old woman developed GIST with multiple liver metastases, and she was treated with imatinib and sunitinib. However, these therapies were discontinued, and regorafenib was administered. Twenty-four months after beginning regorafenib, she developed an acneiform eruption on her back. Histopathologic analysis of a skin biopsy from the back revealed neutrophilic suppurative folliculitis. Therefore, she postponed regorafenib administration for 2 months and was treated with topical application of clindamycin phosphate hydrate, which was effective. Consistent with reported evidence that the presence of acneiform eruption and the efficacy of EGFR inhibitors are positively associated, regorafenib had good anticancer activity in our patient. Ultimately, we found that although regorafenib-associated skin toxicities usually appear within 1 month of treatment, patients potentially can present with delayed-onset acneiform eruptions even 24 months later.
Chamberlain F, Farag S, Williams-Sharkey C, et al. Toxicity management of regorafenib in patients with gastrointestinal stromal tumour (GIST) in a tertiary cancer centre. Clin Sarcoma Res 2020;10:1. DOI: https://doi.org/10.1186/s13569-019-0123-4
Liu YC, Tsai JJ, Weng YS et al. Regorafenib suppresses epidermal growth factor receptor signaling-modulated progression of colorectal cancer. Biomed Pharmacother 2020;128:110319. DOI: https://doi.org/10.1016/j.biopha.2020.110319
Demetri GD, Reichardt P, Kang YK et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:295-302. DOI: https://doi.org/10.1016/S0140-6736(12)61857-1
Brodell LA, Hepper D, Lind A et al. Histopathology of acneiform eruptions in patients treated with epidermal growth factor receptor inhibitors. J Cutan Pathol 2013;40:865-70. DOI: https://doi.org/10.1111/cup.12202
Ansai O, Fujikawa H, Shimomura Y et al. Case of severe acneiform eruptions associated with the BRAF inhibitor vemurafenib. J Dermatol 2017;44:e15-e6. DOI: https://doi.org/10.1111/1346-8138.13534
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.





 https://doi.org/10.4081/dr.2022.9303
https://doi.org/10.4081/dr.2022.9303



