Increased serum interleukin-17A levels correlate with disease severity and poor prognostic factors in patients with alopecia areata
HTML: 3
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
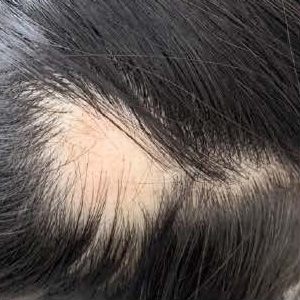
Alopecia areata (AA) is a tissue-specific autoimmune disease characterized by non-scarring and rapid onset of hair loss. Interleukin (IL)-17A is mainly produced by T helper 17 (Th17) cells and may play a crucial role in the pathogenesis of various autoimmune diseases including AA. We conducted this research to measure serum level of IL-17A in patients with AA and investigated its relationship with the clinical manifestations in patients with AA. We assessed 36 patients with AA and 20 healthy control subjects. Demographic information and clinical characteristics were determined by physical examination and via the review of medical history. Serum IL-17A was measured by using enzyme-linked immunosorbent assay. Serum IL-17A concentration was significantly higher in patients with AA than in the control group (P=0.004). The AA patients with severe presentation, personal atopy, nail abnormalities, or active phase had significantly higher serum IL-17A levels compared to others without these signs. Increased serum IL-17A levels in patients with AA correlate with severity and indicate an active disease state. These findings suggest that IL-17A may play an important role in determining the pathogenesis of AA and may serve as a valuable clinical biomarker of this disease.
Trueb RM, Dias M. Alopecia Areata: a Comprehensive Review of Pathogenesis and Management. Clin Rev Allergy Immunol 2018;54:68-87.
Lew BL, Cho HR, Haw S, et al. Association between IL17A/IL17RA Gene Polymorphisms and Susceptibility to Alopecia Areata in the Korean Population. Ann Dermatol 2012;24:61-5.
Atwa MA, Youssef N, Bayoumy NM. T-helper 17 cytokines (interleukins 17, 21, 22, and 6, and tumor necrosis factoralpha) in patients with alopecia areata: association with clinical type and severity. Int J Dermatol 2016;55:666-72.
El-Morsy EH, Eid AA, Ghoneim H, Al-Tameemi KA. Serum level of interleukin-17A in patients with alopecia areata and its relationship to age. Int J Dermatol 2016;55:869-74.
Kavak A, Baykal C, Ozarmagan G, Akar U. HLA in alopecia areata. Int J Dermatol 2000;39:589-92.
You HR, Kim SJ. Factors Associated with Severity of Alopecia Areata. Ann Dermatol 2017;29:565-70.
Seetharam KA. Alopecia areata: an update. Indian J Dermatol Venereol Leprol 2013;79:563-75.
Dainichi T, Kabashima K. Alopecia areata: What’s new in epidemiology, pathogenesis, diagnosis, and therapeutic options? J Dermatol Sci 2017;86:3-12.
Pratt CH, King LE Jr., Messenger AG, et al. Alopecia areata. Nat Rev Dis Primers 2017;3:17011.
Tembhre MK, Sharma VK. T-helper and regulatory T-cell cytokines in the peripheral blood of patients with active alopecia areata. Br J Dermatol 2013; 169:543-8.
Ghilardi N, Ouyang W. Targeting the development and effector functions of TH17 cells. Semin Immunol 2007;19:383-93.
Yu JJ, Gaffen SL. Interleukin-17: a novel inflammatory cytokine that bridges innate and adaptive immunity. Front Biosci 2008;13:170-7.
Li SF, Zhang XT, Qi SL, et al. Allergy to dust mites may contribute to early onset and severity of alopecia areata. Clin Exp Dermatol 2015;40:171-6.
Bain KA, McDonald E, Moffat F, et al. Alopecia areata is characterized by dysregulation in systemic type 17 and type 2 cytokines, which may contribute to disease-associated psychological morbidity. Br J Dermatol 2020;182:130-7.
Madani S, Shapiro J. Alopecia areata update. J Am Acad Dermatol 2000;42:549-70.
Alkhalifah A, Alsantali A, Wang E, et al. Alopecia areata update: part I. Clinical picture, histopathology, and pathogenesis. J Am Acad Dermatol 2010;62:177-90.
Ikeda T. A new classification of alopecia areata. Dermatologica 1965;131:421-45.
Cunliffe WJ, Hall R, Newell DJ, Stevenson CJ. Vitiligo, thyroid disease and autoimmunity. Br J Dermatol 1968; 80:135-9.
Cunliffe WJ, Hall R, Stevenson CJ, Weightman D. Alopecia areata, thyroid disease and autoimmunity. Br J Dermatol 1969;81:877-81.
Seyrafi H, Akhiani M, Abbasi H, et al. Evaluation of the profile of alopecia areata and the prevalence of thyroid function test abnormalities and serum autoantibodies in Iranian patients. BMC Dermatol 2005;5:11.
Krishnaram AS, Saigal A, Adityan B. Alopecia areata - Vitiligo overlap syndrome: an emerging clinical variant. Indian J Dermatol Venereol Leprol 2013;79:535-7.
Walker A, Mesinkovska NA, Boncher J, et al. Colocalization of vitiligo and alopecia areata presenting as poliosis. J Cutan Pathol 2015;42:150-4.
Xin C, Sun X, Lu L, et al. Increased Incidence of Thyroid Disease in Patients with Alopecia Areata: A Systematic Review and Meta-Analysis. Dermatology 2019:1-4.
Noda S, Suarez-Farinas M, Ungar B, et al. The Asian atopic dermatitis phenotype combines features of atopic dermatitis and psoriasis with increased TH17 polarization. J Allergy Clin Immunol 2015;136:1254-64.
Koga C, Kabashima K, Shiraishi N, et al. Possible pathogenic role of Th17 cells for atopic dermatitis. J Invest Dermatol 2008;128:2625-30.
Tosti A, Morelli R, Bardazzi F, Peluso AM. Prevalence of nail abnormalities in children with alopecia areata. Pediatr Dermatol 1994;11:112-5.
Sharma VK, Dawn G, Muralidhar S, Kumar B. Nail changes in 1000 Indian patients with alopecia areata. J Eur Acad Dermatol Venereol 1998;10:189-91.
Kasumagic-Halilovic E, Prohic A. Nail changes in alopecia areata: frequency and clinical presentation. J Eur Acad Dermatol Venereol 2009;23:240-1.
Teraki Y, Imanishi K, Shiohara T. Cytokines in alopecia areata: contrasting cytokine profiles in localized form and extensive form (alopecia universalis). Acta Derm Venereol 1996;76:421-3.
Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 2006;441:235-8.
Tojo G, Fujimura T, Kawano M, et al. Comparison of interleukin-17- producing cells in different clinical types of alopecia areata. Dermatology 2013;227:78-82.
Tabarkiewicz J, Pogoda K, Karczmarczyk A, et al. The Role of IL-17 and Th17 Lymphocytes in Autoimmune Diseases. Arch Immunol Ther Exp (Warsz) 2015;63:435-49.
Guttman-Yassky E, Nia JK, Hashim PW, et al. Efficacy and safety of secukinumab treatment in adults with extensive alopecia areata. Arch Dermatol Res 2018;310:607-14.
Gautam RK, Singh Y, Gupta A, et al. The profile of cytokines (IL-2, IFNgamma, IL-4, IL-10, IL-17A, and IL-23) in active alopecia areata. J Cosmet Dermatol 2020;19:234-40.
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.





 https://doi.org/10.4081/dr.2021.9398
https://doi.org/10.4081/dr.2021.9398



